This content is only available in German.
Dieser Inhalt ist nur auf Deutsch verfügbar.
0900 712 712
(3.23 CHF / min. from the Swiss landline, possibly additionally 8 Rp. / min. in the waiting loop by network operator)
0900 712 713
(3.12 CHF / min. for calls from prepaid cell phones, possibly additionally 8 Rp. / min. in the waiting loop by network operator)
Notfallnummern
Hotline for child and youth emergencies
The Medgate Kids Line provides fast and simple medical advice when your child is unwell. The medical team from our partner Medgate is available by phone around the clock.
0900 712 712
(3.23 CHF / min. from the Swiss landline, possibly additionally 8 Rp. / min. by network operator)
0900 712 713
(3.13 CHF / min. for calls from prepaid cell phones, possibly additionally 8 Rp. / min. by network operator)
Please note: the Medgate Kids Line is currently offered in German.
Important Numbers
- 144 Ambulance
- 145 Tox Center (Poisoning)
- 117 Police
- 118 Fire Department
Kontakt Box
Pediatric Infectious Diseases and Vaccinology
Nicole Ritz's research focuses on enhancing current strategies to diagnose and prevent tuberculosis (TB) in children. This includes immunodiagnostic tests including interferon gamma release assays (IGRA), novel cytokine-based assays and other biomarkers for early detection of childhood TB. Her research interest also focuses on Bacille-Calmette-Guérin (BCG) vaccine the only currently licenced vaccine against TB, in particular on specific and non-specific immune response including adaptive and innate immunity.
Jan Bonhoeffer's research strives to improve child health by preventing infectious diseases and optimizing treatment through improved diagnostics. His focus is on generating best evidence for benefit-risk assessment of vaccines with a global network of partners and health care databases. He is also investigating strategies to optimize antibiotic use by improving the diagnosis of respiratory tract infections in children.
Ulrich Heininger's main research interest lies in the field of pertussis, a respiratory tract disease caused by the bacterium Bordetella pertussis. He and his national and international collaborators are focussing mainly on the epidemiology and prevention of pertussis by vaccination. Further, Ulrich Heininger is working on the general acceptance and impact of immunization per se amongst the public as well as health care workers.
Main research projects
Please note that not every project listed here was necessarily initiated by our group.
ANSORP - A multicenter, multinational serosurvey study for pertussis among children 10-18 years old in Asia
Study goal: This study aims to measure IgG anti-PT in Asian children aged 10-18 years. This will enable us to identify how many children have evidence of a recent pertussis infection defined by IgG anti-PT above 125 IU/mL and how many children are potentially susceptible to pertussis infection when exposed.
Collaborators
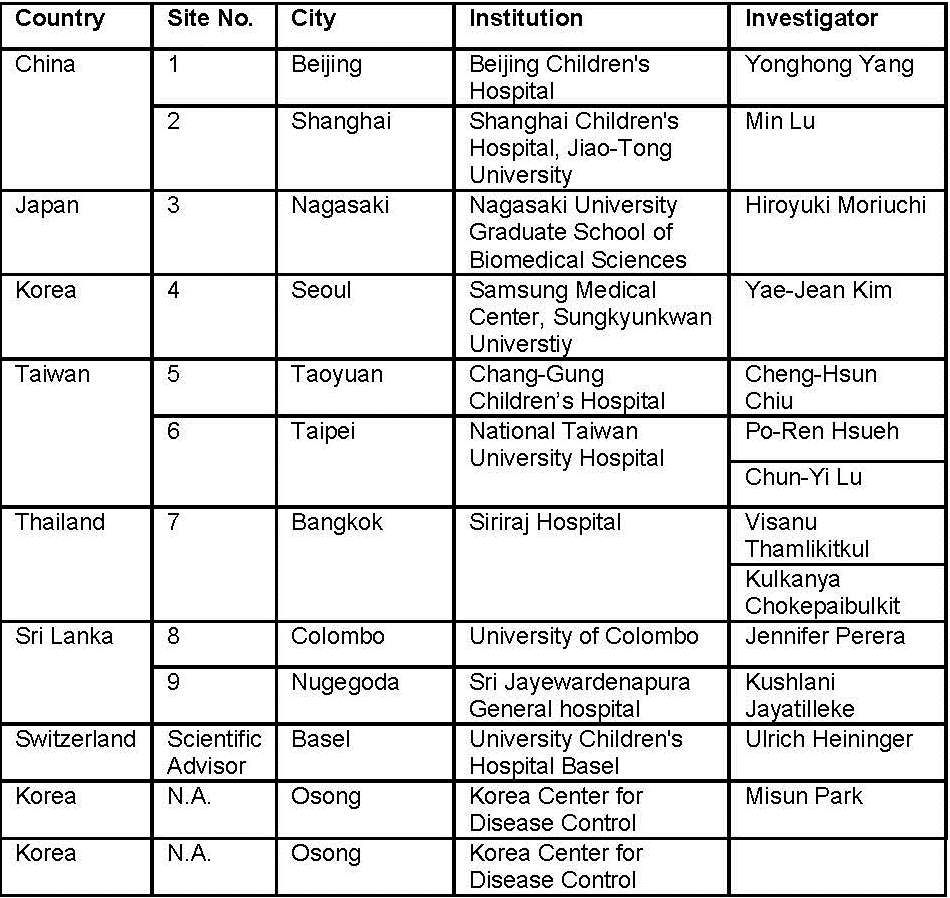
- Timelines: Start: Q3 or 4/2013; Duration: 24 months
- Financial support: SPMSD, Lyon, France
Epidemiology of Pertussis in Switzerland – Hospitalized Cases via SPSU (Swiss Pediatric Surveillance Unit)
- Study goal: To monitor the effect of new pertussis immunization recommendations in Switzerland (adolescents, young adults, and pregnant women) on the epidemiology of pertussis in children. This will be done using assessment of hospitalizations due to pertussis in children (0-15 years of age) within the framework of the Swiss Pediatric Surveillance Unit (SPSU).
- Collaborators: Co-prinicipal investigators: Ulrich Heininger (UKBB) and Jean-Luc Richard (BAG); Further collaborators: all Swiss pediatric hospitals and units (SPSU); Laboratory analyses: IMM; Univ. of Basel
- Timelines: 01/2013 - 12/2016.
- Financial support: BAG
The influence of host, laboratory and environmental factors on the performance of cytokine-based immunodiagnostic tests in children
PERISCOPE – PERtussIS COrrelates of Protection Europe
New Innovative Medicine Initiative (IMI)
2 project on the trail of next generation vaccines against pertussis (whooping
cough)
Despite the availability of
effective prophylactic vaccines against pertussis, there has been a rise in the
incidence of pertussis, with epidemics in Europe, Australia and the US in the
last decade. As well as being a particular problem in vulnerable infants, with
devastating consequences in developing countries, the incidence of pertussis is
also increasing in adolescents and adults, particularly in industrialized
countries. Hence, pertussis continues to be a major public health concern
worldwide.
The pan-European initiative PERISCOPE
sets out to accelerate improvement of prophylactic vaccines and vaccination
strategies for pertussis. The project is funded with a total budget of €28mio
over the next five years by the Innovative Medicines Initiative (IMI), a joint
undertaking of the European Commission and the European Federation of Pharmaceutical
Industries and Associations (EFPIA). Additionally, PERISCOPE is the first IMI
project to receive funding from the Bill & Melinda Gates Foundation.
"Given the resurgence
and changing epidemiology of pertussis in industrialized countries and the
persistent low level of vaccination coverage and high infant mortality caused
by pertussis in low income countries, PERISCOPE represents a concerted effort aiming
to accelerate the development of improved vaccination strategies that ensure
solid, long-lasting protection against infection", says Prof. Ronald de Groot
from Radboud University Medical Center and coordinator of PERISCOPE. "This implies, first
and foremost, that we need to gain a better understanding of the pathogenesis
and underlying mechanisms of the infection to be able to identify novel vaccine
candidates. However, it is equally important to rebuild the ecosystem for
pertussis research and the technical infrastructure needed in Europe to
successfully evaluate those novel candidates".
PERISCOPE will focus
on three major areas:
1) Setting up a comprehensive clinical research programme
to study the immune response of individuals of all ages
to pertussis infection and vaccination; 2) the establishment of parallel
clinical and pre-clinical models of pertussis infection, and 3) the development
of a comprehensive battery of state-of-the-art bioassays, to help reveal the markers of an effective and long-lasting
immune response to pertussis infection in both clinical and preclinical study
subjects. PERISCOPE will also seek to study immunization in pregnancy to gain a
better understanding of the impact of maternal antibodies on the infant’s
immune responses to pertussis.
In the frame of this ambitious project, Prof. Ulrich
Heininger will lead workpackage 4 with the goal to facilitate interaction with experts from regulatory
and public health agencies to ensure acceptance of novel and existing
biomarkers and facilitate improved design of small-scale Phase II/III trials.
PERISCOPE assembles a multi-national team of leading experts from 22 partnering institutions with long-standing experience in pertussis research, clinical trials, bioinformatics, immunology and public health. The consortium also includes Sanofi Pasteur and GlaxoSmithKline, two of the world’s largest and most experienced vaccine manufacturers, as industrial partners.
Partners in PERISCOPE
Belgium
- GlaxoSmithKline
- Q-Biologicals NV
- Université libre de Bruxelles
Czech Republic
- Mikrobiologicky
USTAV - AVCR, V.V.I.
Finland
- Turun Yliopisto
France
- Commissariat à l’énergie atomique et aux energies alternatives
- Institut Pasteur de Lille Fondation
- Sanofi
Pasteur SA
The Gambia
- Medical Research Council
Germany
- Eurice GmbH
Ireland
- Trinity College Dublin
Netherlands
- Leids Universiteir Medisch Centrum
- Radboud university medical center
- Rijksinstituut voor
Volksgezondheid en Milieu part of the Dutch State
Spain
- Universidad de Salamanca
Switzerland
- Universität Basel
- Hospices Cantonaux CHUV
UK
- The Chancellor, Masters and Scholars of the University of Oxford
- Department of Health (Public Health England)
- Imperial College of Science Technology and Medicine
- University of Bath
- University of Southampton
Project details
Name: PERISCOPE – PERtussIS COrrelates
of Protection Europe
Start date: 2016-03-01
Duration: 60 months
Budget: €28mio
Coordination: Stichting
Katholieke Universiteit, Radboud university medical center
Contact
Coordinator
- Radboud university medical center
- Prof. Ronald de Groot
- Phone: +31 24 366 73 47
Project Management
- Eurice - European Research and Project Office GmbH
- Dr. Claudia Schacht
- Phone: +49 30 370 44 15 831
EFPIA Coordinator
- Sanofi Pasteur
- Dr. Patricia Londoño-Hayes
More information about the project
- Epidemiology of tuberculosis in Swiss children
- Improving the diagnosis of tuberculosis in children: combining cytokine-based immunodiagnosis with direct and indirect detection of Mycobacterium tuberculosis
- Improving the reading of tuberculin skin tests
Group members
Prof. Ulrich Heininger, MD
Senior Physician / Dep. Head of Paediatrics / Head of Paediatric Infectiology and Vaccinology
Infectiology / Vaccinology, Infection Prevention
Prof. Dr. med. Ulrich Heininger - HR - Our staff - UKBB an independent, university-wide competence center for pediatric and adolescent medicine. Contact us!
Julia Bielicki, MD, PhD
Senior Physician Pediatrics
& Pediatric Infectious Diseases
Medical Coordinator PRCDr. med. Julia Bielicki - HR - Our staff - UKBB an independent, university-wide competence center for pediatric and adolescent medicine. Contact us now!
Prof. Jan Bonhoeffer, MD
Professor of Pediatrics, Infectious Diseases and Vaccines
Research Group HeadProf. Dr. med. Jan Bonhoeffer - HR - Our staff - UKBB an independent, university-wide competence center for pediatric and adolescent medicine. Contact us!
- Chloé Schläppi, MD
- Nina Vaezipour, MD
- Rahel Erlacher, MD
- Michael Büttcher, MD
Emergencies
0900 712 712
(3.23 CHF/min. CH-landline, possibly additionally 8 Rp. / min. by network operator)
0900 712 713
(3.12 CHF/min. prepaid cell phones, possibly additionally 8 Rp. / min. by network operator)
Contact
University Children’s Hospital Basel
Spitalstrasse 33
4056 Basel / Switzerland
T +41 61 704 12 12
Contact
About us
Legal Requirements
Emergency
Contact
University Children’s Hospital Basel
Spitalstrasse 33
4056 Basel / Switzerland
T +41 61 704 12 12
Contact
© UKBB, 2024






