This content is only available in German.
Dieser Inhalt ist nur auf Deutsch verfügbar.
0900 712 712
(3.23 CHF / min. from the Swiss landline, possibly additionally 8 Rp. / min. in the waiting loop by network operator)
0900 712 713
(3.12 CHF / min. for calls from prepaid cell phones, possibly additionally 8 Rp. / min. in the waiting loop by network operator)
Notfallnummern
Hotline for child and youth emergencies
The Medgate Kids Line provides fast and simple medical advice when your child is unwell. The medical team from our partner Medgate is available by phone around the clock.
0900 712 712
(3.23 CHF / min. from the Swiss landline, possibly additionally 8 Rp. / min. by network operator)
0900 712 713
(3.13 CHF / min. for calls from prepaid cell phones, possibly additionally 8 Rp. / min. by network operator)
Please note: the Medgate Kids Line is currently offered in German.
Important Numbers
- 144 Ambulance
- 145 Tox Center (Poisoning)
- 117 Police
- 118 Fire Department
Kontakt Box
KIDS-STEP
The aim of the KIDS-STEP trial is to investigate if anti-inflammatory medication (steroids) positively affect the severity of illness in children with lower airway infections (chest infection, pneumonia). This will be investigated in children admitted to hospital with a chest infection who receive standard therapy.
The KIDS-STEP trial investigates if we can observe
- a less severe disease
- a shorter duration of illness
- a shorter hospital stay
- less complications
in children who receive steroids.
KIDS-STEP-Studie: deutsche Version
KIDS-STEP STUDY english version
L'édude KIDS-STEP: version française
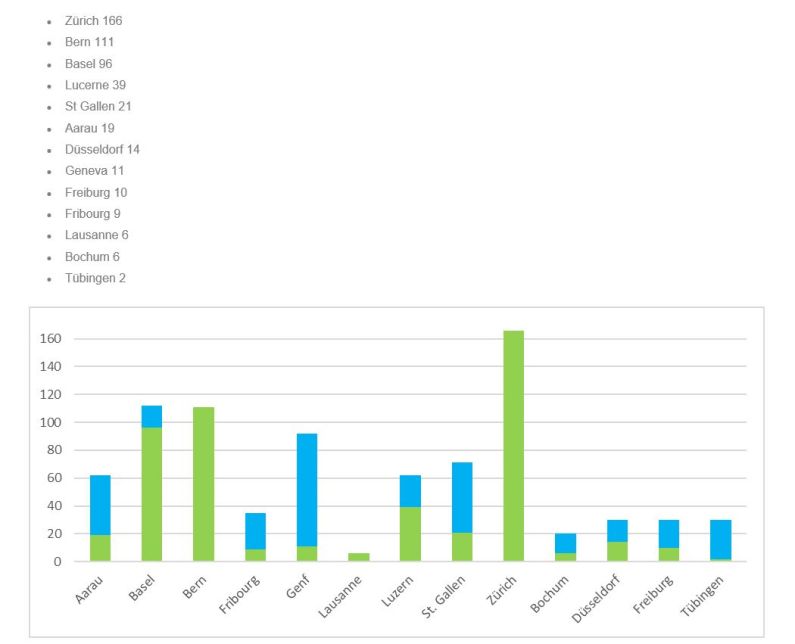
Participating hospitals in Switzerland
Aarau
Principal Investigator Aarau
Prof. Dr. med. Henrik Koehler
Oberarzt päd. Pneumologie
Kantonsspital Aarau AG, Klinik für Kinder u. Jugendliche, Tellstr. 25, 5001 Aarau
T +41 62 838 92 44
Co-Investigator Aarau
Patrick Haberstich
Study Team Aarau
Rachel Kusche
Sandra Knecht
Basel
Principal Investigator Basel
Prof. Dr. med. Ulrich Heininger, MD
E-Mail
Study Team Basel - Pediatric Research Centre (PRC)
Claudia Werner
Britta Wagner
Alexandra Meyer
Artemis Ioannaki
Bern
Principal Investigator Bern
Kristina Keitel
Inselspital Bern, Notfallzentrum für Kinder und Jugendliche
Freiburgstrasse 15, 3010 Bern
Study Team Bern
Verena Wyss
Barbara Beck
E-Mail
Fribourg
Principal Investigator Fribourg
Dr. Petra Zimmermann
Pädiatrie
- HFR Freiburg – Kantonsspital
Chemin des Pensionnats 2-6 | 1752 Villars-sur-Glâne
T +41 26 306 35 10
Study
Nurse Fribourg
Julie Tomasini
T +41 26 306 35 98
Genf
Principal Investigator Genf
Dr. med. Anne Mornand
Geneva University Hospital, Department of Pediatrics
rue Willy Donzé 6, 1211 Geneva 14
T +41 22 372 45 79
Co-Investigator Genf
Klara Posfay-Barbe
Research Coordinator Genf
Renato Gualtieri
Luzern
Principal Investigator Luzern
Marco Lurà, MD
Oberarzt Pädiatrie
Luzerner Kantonsspital, Kinderspital, Spitalstrasse, 6000 Luzern 16
T +41 41 205 68 38
M +41 79 550 89 38
Co-Investigator Luzern
Alex Donas
Study Team Luzern
Katja Hrup
Janine Stritt
St. Gallen
Principal Investigator St. Gallen
Dr. Christian Kahlert
Oberarzt Infektiologie
Claudiussstrasse 6, 9006 St. Gallen
T +41 71 243 14 34
Study Coordinator St. Gallen
Ingrid Beck
T +41 71 243 19 67
Zürich
Principal Investigator Zürich
Christoph Berger, MD
Leiter Abteilung Infektiologie und Spitalhygiene
Universitäts-Kinderspital Zürich – Eleonorenstiftung, Steinwiesstrasse 75, 8032 Zürich
T +41 44 266 78 40 (direkt)
T +41 44 266 72 50 (Sekretariat)
F +41 44 266 80 72
Co-Investigator Zürich
Dr. med. Patrick Meyer Sauteur
Oberarzt Infektiologie und Spitalhygiene
T +41 44 266 78 96
Participating hospitals in Germany
Bochum
Principal Investigator Bochum
Dr. med. Stefanie Dillenhöfer
Oberärztin Pädiatrische Pneumologie
Universitätsklinikum
der Ruhr-Universität Bochum
Klinik für Kinder-
und Jugendmedizin der Ruhr-Universität Bochum
Alexandrinenstr.
544791 Bochum
Co-Investigator
Bochum
Dr. med. Anne Schlegtendal
Fachärztin
Study Team
Bochum
Dr. med. Anna Wiemers
Sub Investigator
Study Nurses:
Sandra Böger
Michaela Schwarzbach
Veronika Baumeister
Barbara Streich
Düsseldorf
Principal Investigator Düsseldorf
Dr. med. Dirk Schramm
Oberarzt
Bereich Kinder-Pneumologie und Allergologie
Universitätsklinikum Düsseldorf
Moorenstrasse 5
40225 Düsseldorf
T +49 211 81 00
Study Team Düsseldorf
Dr. rer. nat. Nadja Drusenheimer
Dr. rer. nat. Anne Brückner
Freiburg
Principal Investigator Freiburg
Prof. Dr. Markus Hufnagel
Leitung Kinder- und Jugendrheumazentrum
Stv. Leitung Pädiatrische Infektiologie und Rheumatologie, Immunologie
Klinik für Allgemeine Kinder- und Jugendmedizin
Postadresse: Mathildenstrasse 1 / Anfahrt: Heiliggeiststrasse 1
79106 Freiburg
T +49 761 270 45 29 2
Co-Investigator Freiburg
Dr. Roland Elling
Study Coordinator Freiburg
Bianca Rippberger
Tübingen
Principal Investigator
Tübingen
Dr. Tobias Walter
Oberarzt
Facharzt für Kinder und
Jugendmedizin, Zusatzbezeichnung Kinder-Pneumologie
Notaufnahme,
Asthma/Allergiesprechstunde
T +49 7071 29-83781
Co-Investigator Tübingen
Dr. med. Hanna Renk
Funktionsoberärztin, Fachärztin für
Kinder- und Jugendmedizin
Pädiatrische Infektiologie; Pädiatrische Intensivmedizin
Universitätsklinikum Tübingen, Postfach
2669, 72016 Tübingen
T +49 7071 29-62250
Study Team Tübingen
Annette Hark
T +49 7071 29-81397
Lung infections are a common reason for children having to stay in hospital. Based on already known information on the effect of cortisone in lung infections, the severity of the disease in children may be reduced by cortisone and the hospital stay of children may be shortened. In children, there is still no significant data for the beneficial use of cortisone in lower respiratory tract infections. This is why we would like to evaluate this in our trial.
Participation is open to all children who are admitted to hospital (at a participating center) for a chest infection and who are between 6 months and 13 years of age and weigh between 5 and 45 kilograms.
Children are prohibited from participation if they suffer from a severe chronic condition (e.g. mucoviscidosis), have a severe complication (e.g. a large fluid collection in their chest) or if they are known to be allergic to betamethasone or any additional ingredients in Celestamine®.
KIDS-STEP is a randomised, placebo-controlled and double-blind trial. This means that the participating children are randomly split into two groups (Celestamine® group and placebo group). Children in the Celestamine® group receive medication that contains the active ingredient and children in the placebo group receive medication that does not contain an active ingredient. A child will be randomly assigned to one of the groups (randomisation). This means that it is due to chance whether a child will receive Celestamine® or placebo. Neither the parents nor the trial team can decide which group a child will be assigned to. The chance to be assigned to each group is the same (50%).
Irrespective of group allocation a nasal swab will be taken from every participating child on day 1 and 3. For this a cotton bud will be placed in the child’s nose for a few seconds to collect nasal secretions. These nasal secretions can then be tested in the lab for viruses and bacteria that can be associated with lower airway infections.
After regular discharge from hospital a trial team member will call a family member 1, 2 and 4 weeks after joining the trial and will ask a few questions about the child’s health and wellbeing. After the call in week four the child‘s participation ends.
Several hundred children in Switzerland and Germany will participate in the KIDS-STEP study throughout the duration of the study.
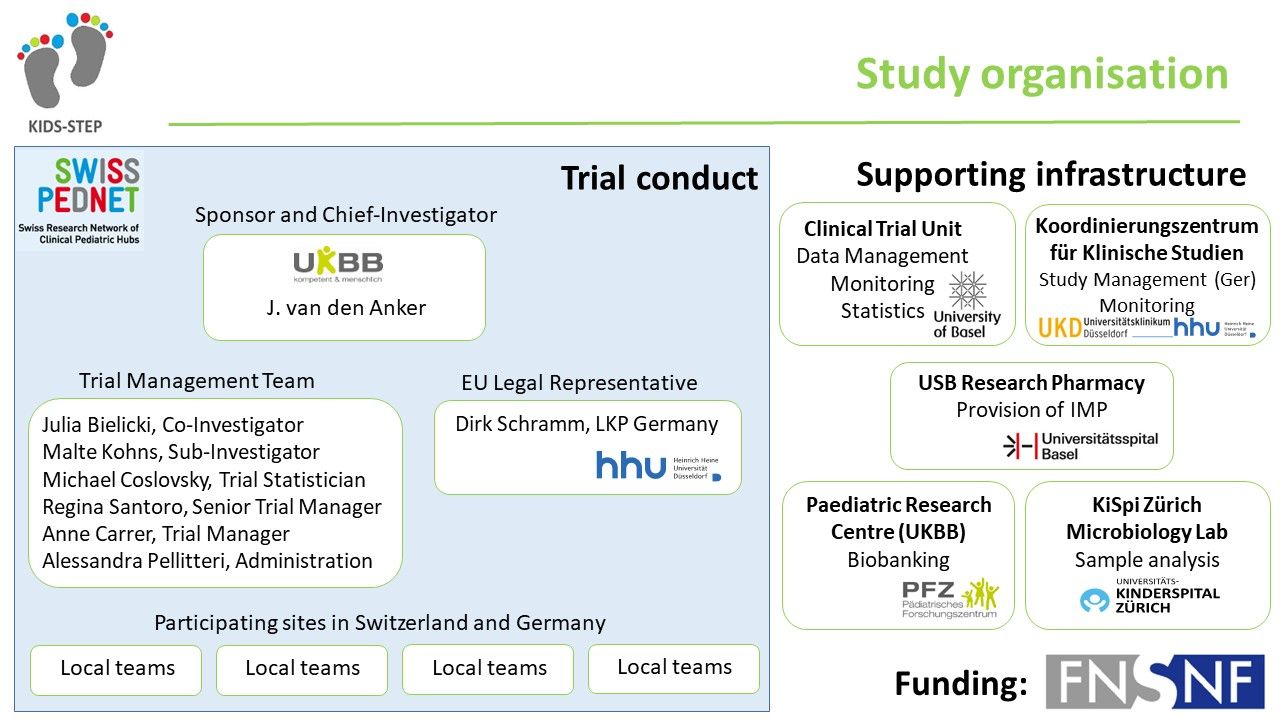
The KIDS-STEP study is carried out within the SwissPedNet research network. SwissPedNet coordinates and supports high-quality research in child health. All major children's hospitals and university children's hospitals in Switzerland will participate in the KIDS-STEP study.

Contact
Prof. Johannes van den Anker, MD, PhD
Research Group Leader, Senior Physician
Head of Pediatric Pharmacology
Prof. Dr. med. Johannes van den Anker - Personal - Das UKBB ist ein universitäres Kompetenzzentrum für Kinder- und Jugendmedizin. Ihre Experten kontaktieren!
Julia Bielicki, MD, PhD
Senior Physician Pediatrics
& Pediatric Infectious Diseases
Medical Coordinator PRCDr. med. Julia Bielicki - HR - Our staff - UKBB an independent, university-wide competence center for pediatric and adolescent medicine. Contact us now!
Malte Kohns Vasconcelos, MD
Attending Physician, Scientific researcher
Immunology, Infectiology, Pharmacology
Malte Kohns - HR - Our staff - UKBB an independent, university-wide competence center for pediatric and adolescent medicine. Contact your med experts now!
Regina Santoro
Senior Physician KSA, Co-Leader
Team Leader PRC
Senior Trial Manager KIDS-STEPRegina Santoro - HR - Our staff - UKBB an independent, university-wide competence center for pediatric and adolescent medicine. Contact your med experts now!
Alessandra Pellitteri
Junior Project Manager - Finance
Junior Project Manager - Finance PRC
UKBB ein universitäres Kompetenzzentrum für Kinder- und Jugendmedizin - Lehre - Forschung. Ihre Experten kontaktieren!
Emergencies
0900 712 712
(3.23 CHF/min. CH-landline, possibly additionally 8 Rp. / min. by network operator)
0900 712 713
(3.12 CHF/min. prepaid cell phones, possibly additionally 8 Rp. / min. by network operator)
Contact
University Children’s Hospital Basel
Spitalstrasse 33
4056 Basel / Switzerland
T +41 61 704 12 12
Contact
About us
Legal Requirements
Emergency
Contact
University Children’s Hospital Basel
Spitalstrasse 33
4056 Basel / Switzerland
T +41 61 704 12 12
Contact
© UKBB, 2024